Question: How does an Acid Buffer work?
An acidic buffer is a solution which is able to maintain the pH of the solution (at an acidic level) even if small amounts of base or acid is added.
In every acid molecule, there is a hydrogen atom as part of the molecule. When dissolved in water, the acid molecule would dissociate, forming a $H_3O^+$ ion and a counter ion which is the remaining part of the molecule. However, if the bond between the hydrogen atom and the acid molecule is strong, the counter ion formed would be quite reactive and would easily revert back to its original form. This is where it may “reclaim” a hydrogen atom (either form a $H_2O$ or $H_3O^+$ molecule) and reform the acid molecule. Hence, in weak acids, the bond between the hydrogen atom and the molecule are quite strong , and therefore the counter ion formed would also be reactive, wanting to reform the acid molecule.
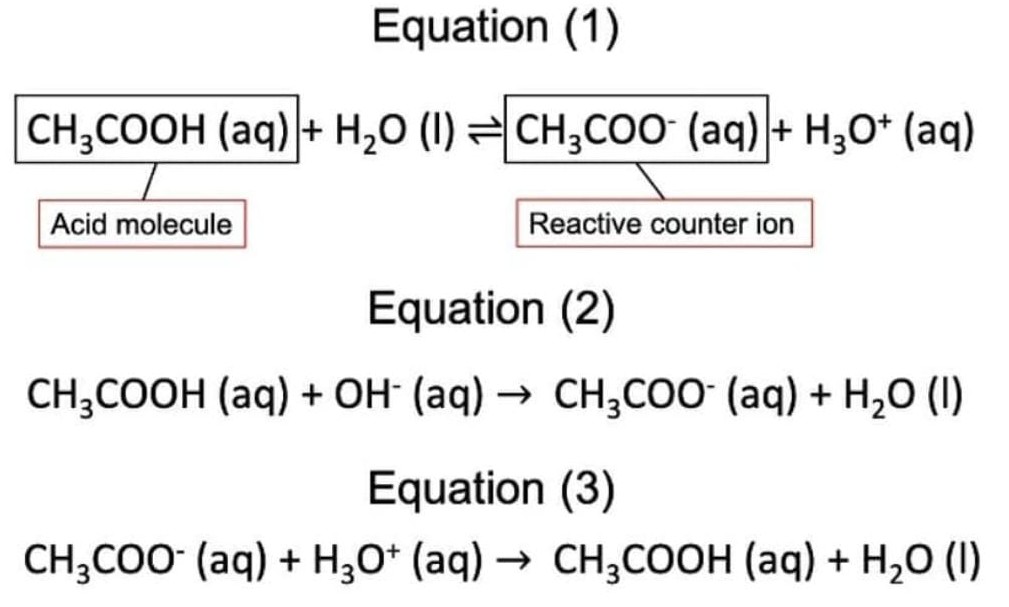
Therefore, when only a weak acid is dissolved in water, the acid molecule would dissociate in water, forming acidic $H_3O^+$ ions as well as the reactive counter ion. The counter ion would then also continually react to reform the acid molecule, forming a reaction of dynamic equilibrium. This is called the natural partial dissociation of a weak acid, shown through the example of ethanoic acid in Equation(1).
This dynamic equilibrium is the basis behind the concept of a buffer, where optimally equal amounts of the acid and its counter ion is added in to the solution, such that the partial dissociation of the weak acid can be suppressed, and an artificial equilibrium can be established.
When $OH^-$ ions (a base) are added to such a solution, the base would forcefully break the bond between the hydrogen and the acid molecule, forming the counter ion and water. This is shown through Equation(2). This would remove the $OH^-$ ions from solution and forming the counter ion in the process. Conversely, when $H_3O^+$ ions (an acid) are added to the solution. the counter ion, being reactive, would then help to neutralise the solution by removing the hydrogen atom from $H_3O^+$ and reforming water and the acid, as shown through Equation(3).
Therefore, this results in a solution of relatively constant pH, as any additions of $H_3O^+$ or $OH^-$ would be removed by the counter ion or the acid respectively.
