What is the difference between $S_N1$ and $S_N2$ reaction mechanism in Nucleophilic substitution?
Hello, just to preface this post the question given is rather general and conceptual, and as such the post would be quite long but I will try to break it up neatly as much as I can. In an attempt to stay focused, I may gloss over certain concepts which may require pre-existing knowledge about organic chemistry, but if you do not understand anything I have said, feel free to dm me! :)
Firstly, Nucleophilic substitution. To give a short description, it is the “replacement” (substitution) of a functional group in the target organic compound with an electron pair donator (nucleophile). The functional group stated may range from hydroxyl to halogen functional group, for the purposes of this explanation I will use halogens, denoted by X.
Mechanisms
So SN1 and SN2 reactions are two mechanisms where this reaction can take place by, each with different reaction pathways. These two reaction pathways both end up with the same products, but they are in constant competition with each other, and which pathway predominates in a reaction varies based on a number of reaction conditions. Hence, I shall not cover the reaction conditions in this post, and only cover concepts which I feel are essential to understanding the mechanisms and the differences between them. So first let me describe the two mechanisms.
$S_N1$
There are two steps in this mechanism. First, is the formation of a carbocation, where the functional group leaves the organic compound. Using a general example of a halogenoalkane RX, where R be a generic carbon chain and X be any halogen group, the step is shown below.
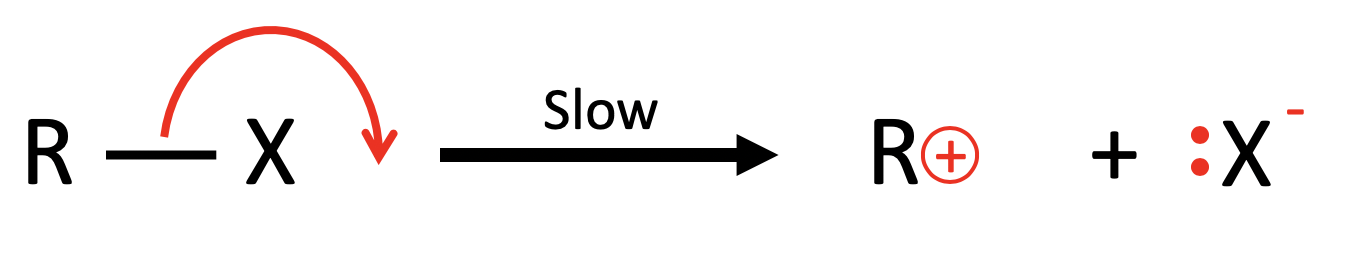
As shown in the diagram, R-X will undergo heterolytic fission (uneven bond breaking) and form two products, a carbocation $R^+$ and the halogen anion $X^-$. Here a functional group (the halogen) has “left” the compound, and hence this functional group is commonly known as the leaving group of any nucleophilic substitution. This is the rate determining step (RDS) of this mechanism, as shown by the “slow” in the diagram. It means that the rate of this step would determine the rate of the entire reaction, and as such the rate equation for this mechanism is Rate = k[RX]. This is a unimolecular (involving one molecule) rate equation, which gives rise to its name $S_N1$.
Here what is important to take note is the formation of a carbocation, a $C^+$ atom. Since this molecule is very unstable, a lot of energy has to be supplied in order to break the R-X bond and form it. Hence, it could be said that if the carbocation formed is stabilised by inductive effects or hyperconjugation, the amount of energy required to break the bond can be reduced. It is this huge amount of energy required to form the carbocation which results in this step being the RDS of the reaction, and by extension it can therefore be said that the stability of the carbocation plays a huge role in determining the rate of a $S_N1$ reaction.
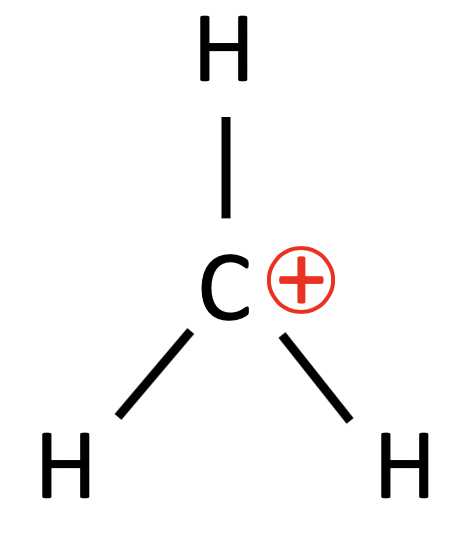
The second step is the “attack” of the nucleophile, where the nucleophile (Nu) reacts and forms a bond with the carbocation and completing the nucleophilic substitution reaction.
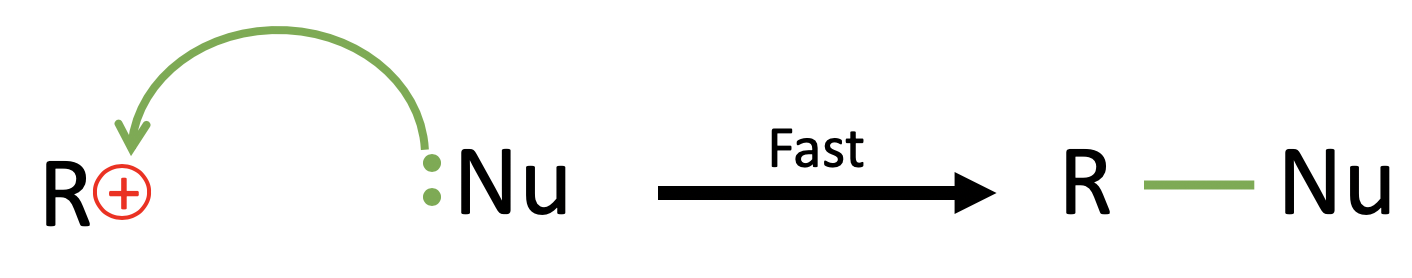
The step is shown as above, and it is shown to be a fast step as it is the formation of a bond rather than the breaking of a bond in the RDS.
What is important here I feel is the structure of the carbocation. As shown through the 3-D structure of the carbocation, it has three electron domains, and hence a trigonal planar geometry. This is significant as it means that it is within a single plane, and that the nucleophile can approach from the front or behind to attack the carbocation.
This is significant as if the three substituents of the carbocation were different and if the nucleophile added was also different from the other 3 substituents, there would be the possibility of the formation of a racemic mixture. This is barring all other possible steric factors affecting the ability of the nucleophile, there would be an equal probability of the nucleophile attacking from the front or back of the carbocation. This would result in the formation of a 1:1 ratio of a pair of enantiomers, also known as a racemic mixture.
$S_N2$
There is only one step in this mechanism, where the nucleophile attacks the carbon atom while the halogen leaves at the same time. Since there is only one step, there is no “slow” or “fast” step in this reaction and the activation energy of this one step would just determine the rate of the whole reaction. Using the same example of a halogenoalkane and a nucleophile in the diagram below, both molecules are actively involved in the one step of the reaction. Hence the rate of the reaction would be determined by both molecules, forming a bimolecular rate equation Rate = k[Nu][RX]. Similar to the reason behind the name of $S_N1$, $S_N2$ is named after its two molecules in the rate equation.
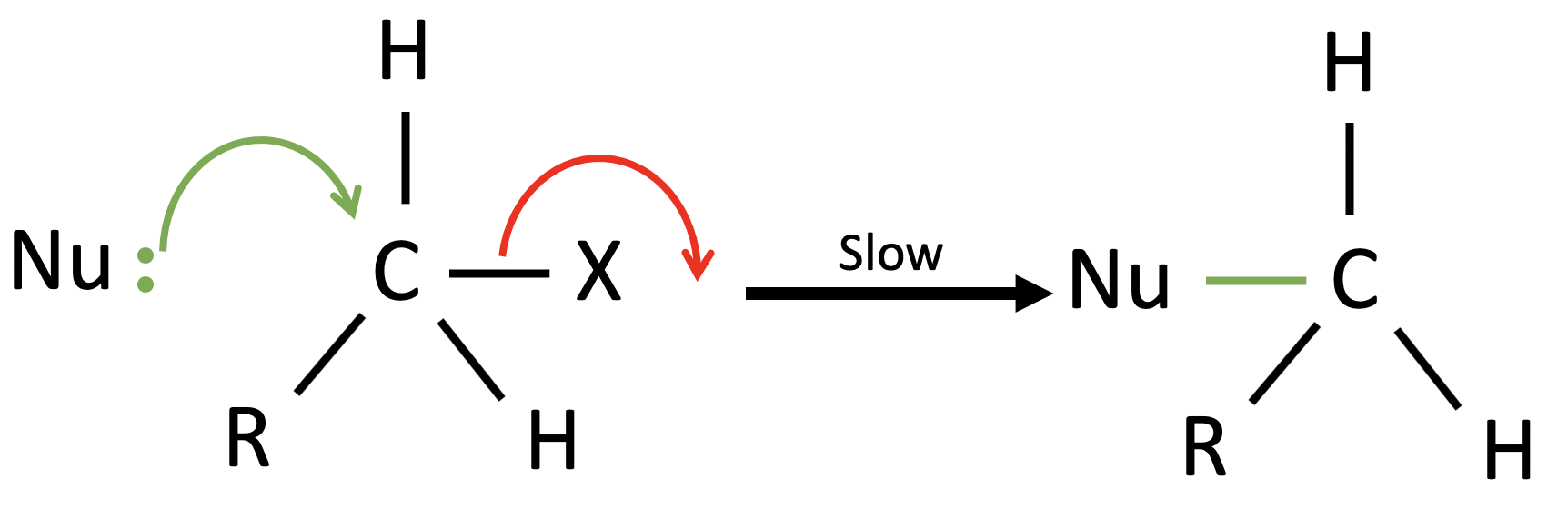
What is significant here is the flip in stereoisomerism here in the reaction. As shown in the step above the nucleophile can only attack from the “back” of the carbon atom, as the halogen is leaving from the “front” of the carbon atom. This is why this is commonly termed as the “backside attack” of the nucleophile. Another thing to take note is my use of inverted commas in the description of the “back” and “front”. This is because I want to bring a point which people may have misconceptions about, and that is this structure is 3 dimensional rather than fixed to a single plane, as it has a tetrahedral structure (4 electron domains). Therefore, in an $S_N2$ reaction after the backside attack, there will be an isomerism flip which occurs as shown in the picture below.
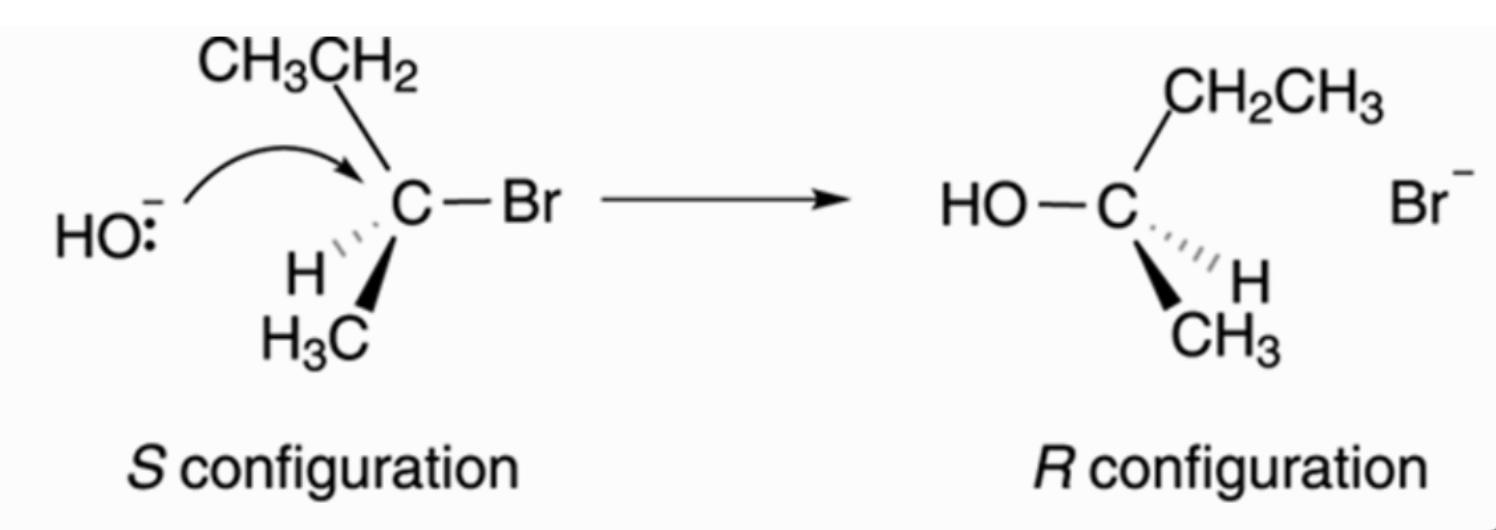
Side note: As I am not a good drawer so I have taken this image off the internet, I will include this link at the end of this post! It is also a good reference website to understanding $S_N1$ and $S_N2$ reactions :)
For those who may have studied the $S_N2$ reaction, I know some of you have seen another structure in the reaction pathway. That is the transition state of this reaction. I have separated them on purpose to illustrate a point that I have, the differences between a transition state and an intermediate. An intermediate by definition is an unstable chemical species which can be isolated experimentally detected and characterised. Meanwhile, a transition state is a structure of highest energy in the middle of any step of a reaction which it cannot be isolated.
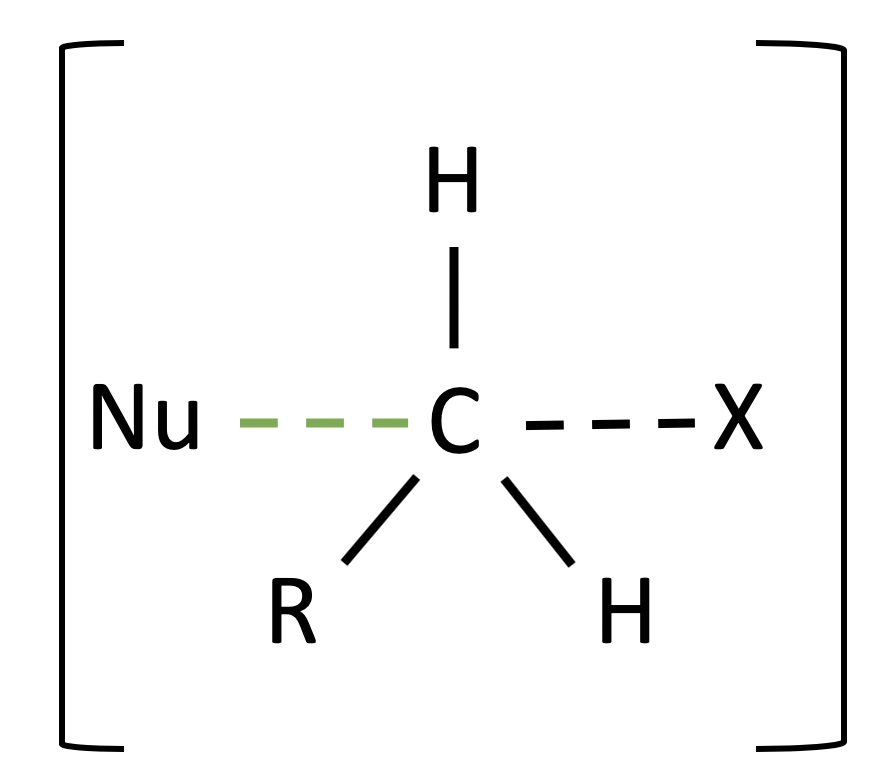
In $S_N1$, there is an intermediate of the carbocation atom, as it is the product of the first step and also used in the second step to complete the reaction. However here in a $S_N2$ reaction there is no intermediate, but rather a transition state which occurs in the process of the reaction. This structure is known as a pentacoordinate transition state, where the structure seems to be a mixture of the structures of both the products and the reactants. As shownabove, this is the transition state of the of the $S_N2$ reaction, and it should be included as part of the reaction pathway. However, one should keep in mind that it is still one step and that there should not be a “slow” or “fast’ notation in your reaction pathway.
However, even though an $S_N2$ does not have a reaction intermediate, it does not mean that an $S_N1$ reaction has no transition states. This brings me to my next part on contrasting the energy profile diagrams of both mechanisms. First, looking at the energy profile for a $S_N1$ reaction, there are both transition states (denoted by TS1 and TS2) and a reaction intermediate (in green). However, in an $S_N1$ reaction, there is only one transition state no intermediates.
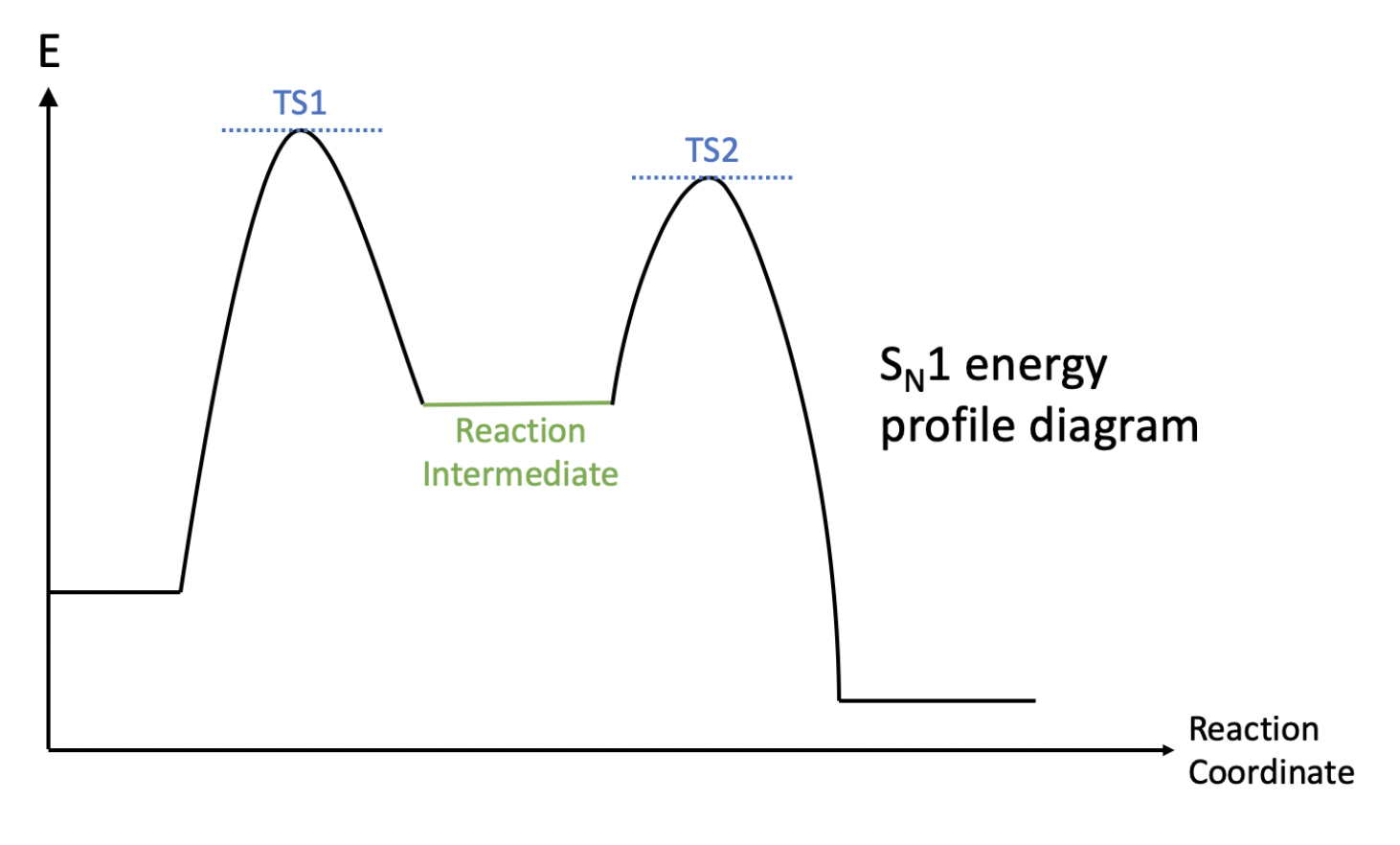
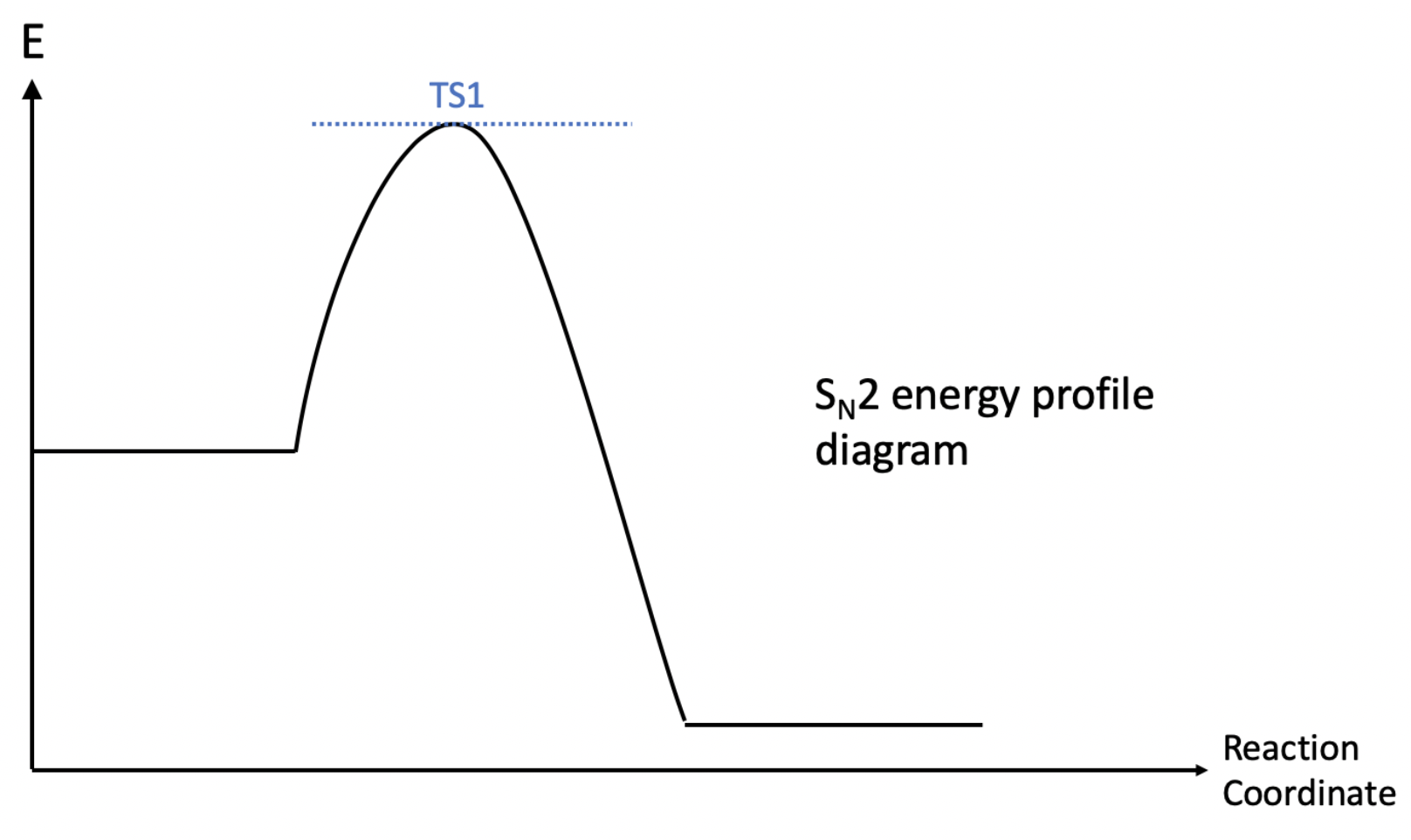
To conclude, in this post I have outlined the main mechanism and differences between a $S_N1$ and $S_N2$ reaction. However, this information is non-exhaustive, and there are many parts which I have glossed over (such as the conditions which favours either reactions). Furthermore, in the process of writing this post I did reference quite a few websites, all of which I will put below.
My References
- https://schoolbag.info/chemistry/organic_1/13.html
- https://www.organicchemistrytutor.com/what-is-the-difference-between-a-transition-state-and-an-intermediate/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_-A%22Carbonyl_Early%22Approach(McMichael)/24%3A_Nucleophilic_Substitution%2C_SN2%2C_SN1
