As we all know, elements all have a nucleus and electrons around it. And in a covalent bond, a pair of electrons is shared between two elements, with electrostatic forces of attraction between the positively charged nuclei and the shared pair of electrons. However, in order to grasp the concept of electronegativity, one must think of electrons as “clouds” rather than as individual discrete particles. Even though there are two electrons in a single covalent bond, it should be seen as a cloud localised over the areas between the two molecules. Therefore, to explain electronegativity in layman terms, it is how much a nucleus of an element “wants” or draws the electron cloud towards it.
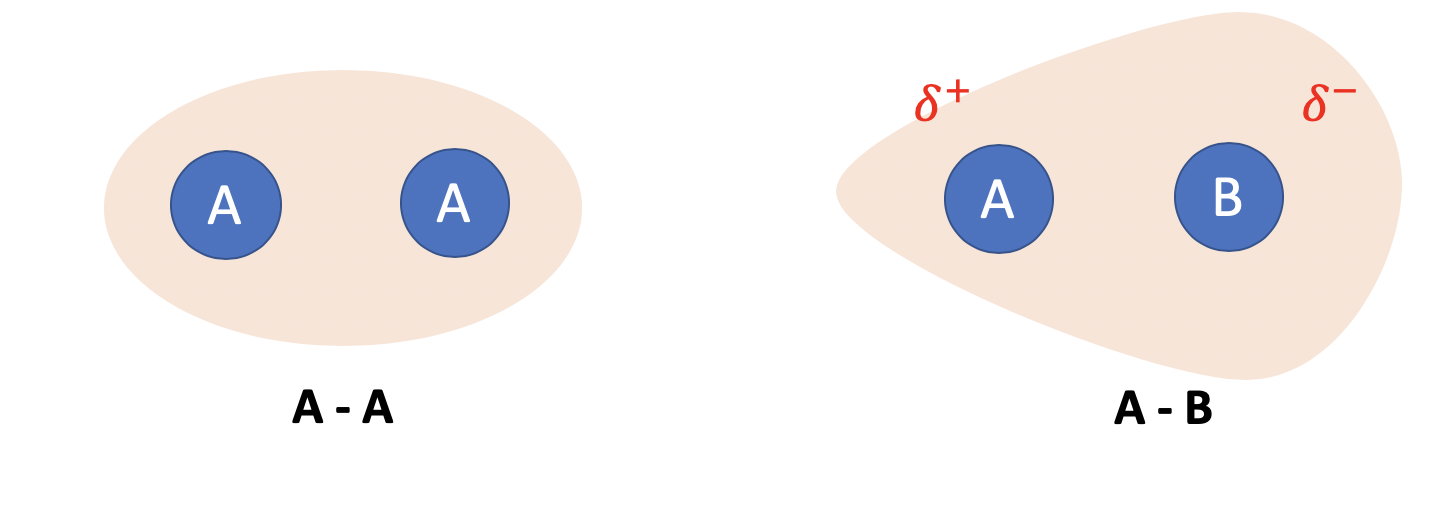
So, to diagrammatically explain what I mean. let’s assume A is bonded to itself. Since they are the exact same molecule, they would both “want” the electron cloud to the same extent, so the electron cloud would mostly remain evenly distributed between the two atoms. This is shown in the picture below, letting the light red area be the electron cloud and the blue circles to be the nuclei of the respective atoms. This is a case of equal electronegativities, where both nuclei “want” the electron cloud the same amount.
Alternatively, let’s say that B is more electronegative than A, and B is bonded to A. In a bond B would “want” the electron cloud more, and hence draw the electron cloud (or electron density) towards it. This is also shown in the picture. Since there are more “electrons” around B and electrons are negatively charged, this will cause the area around B to become partially negatively charged, usually denoted by $\delta^+$. Conversely, the area around A would become partially positively charged, denoted by $\delta^-$. This is factor is important in topics such as organic chem, periodicity and bonding.