
How does bonding work? How do you identify the type of bond in a compound if there are more than three elements?
I think what’s Important here is that what specifically is a bond. A bond is a force of attraction between two atoms in the periodic table, but the type of attraction varies from bond to bond. So, if there are more than two atoms in a compound, you would have to look at the specific atoms present and see what kind of bonds they would form. Let’s use question 3(b) as an example, where it is asking for the bonding within the molecule CH4. However, there are 5 atoms in the molecule, so there has to be more than one bond present in the molecule (since a bond is always only between two atoms). Looking at the periodic table, carbon is in group 14, while hydrogen is in group 1. Hence, a hydrogen atom would have one valence electron and a carbon atom would have 4 valence electrons. Knowing the concept of ionic and covalent bonding, a hydrogen atom would have to gain 1 electron to gain full octet structure (remember lowest energy level only contains 2 electrons), and a carbon atom would have to gain 4 electrons to gain full octet structure. Looking at the molecular formula of CH$_4$, you would hence be able to deduce that one carbon atom would able to gain octet structure if it “gains” 1 electron from each of the 4 hydrogen atoms. Similarly, if a hydrogen atom “gained” one electron from a carbon atom, it would be able to gain octet structure. Hence you can deduce that the carbon forms 4 bonds with the 4 hydrogen atoms, as shown in the picture. Now it is time to determine the type of bond, once you have determined its structure. I think you have missed out this step in determining the type of bonding within a molecule, which is crucial to understanding how bonding works.
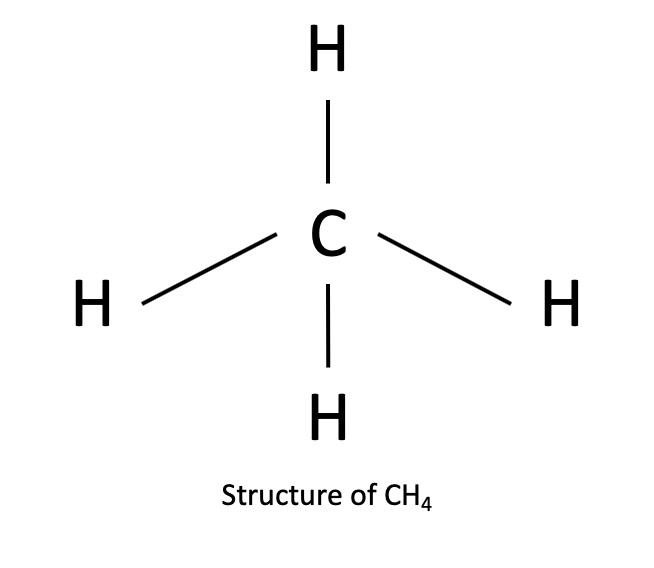
Looking at the electronegativity of the atoms, carbon and hydrogen have an electronegativity of 2.6 and 2.2 respectively, and hence with the difference being 0.4, you would hence be able to determine that it is a covalent bond. Depending on the electronegativity difference between the atoms, you would then be able to determine if each bond is polar covalent, covalent or ionic.
However, such as in question 3(a), it is important that you realise that there may be situations where covalent compounds ($NO_2^-$) acts as an anion in an Ionic Compound. In this case, the question is rather vague as to what kind of bonding it is asking for, but I would assume that it is only considering the cation Ba$^{2+}$ and anion NO$_2^-$ and the bond present is hence ionic in nature.
Lastly, there is also the case of metallic bonding, where it is defined as the electrostatic force of attraction between metal nuclei and a sea of delocalised electrons. Delocalised electrons are common in all metals (and hence their electrical conductivity), and such bonding is only present in metallic alloys, and pure metals, which is quite easy to differentiate from the rest. So, I hope I have clarified your misconceptions and answered your question, if you want the answers to these questions I have them in the next picture but I would suggest that you try on your own before only using my answers as an reference : )
a. Ionic Bonds (assumption stated in answer)
b. Covalent bonds
c. Covalent bonds
d. Metallic Bonds
e. Polar and non-polar covalent bonds
i. For this question I have realised that it is difficult to determine the exact structure of the molecule, and as such I think it is possible to assume that there are polar covalent bonds (as nitrogen is an electronegative atom and most bonds. Formed would be polar), and non-polar covalent bonds (there is carbon and hydrogen present, which would form non-polar covalent bonds as mentioned in my answer)
f. Metallic Bonds
