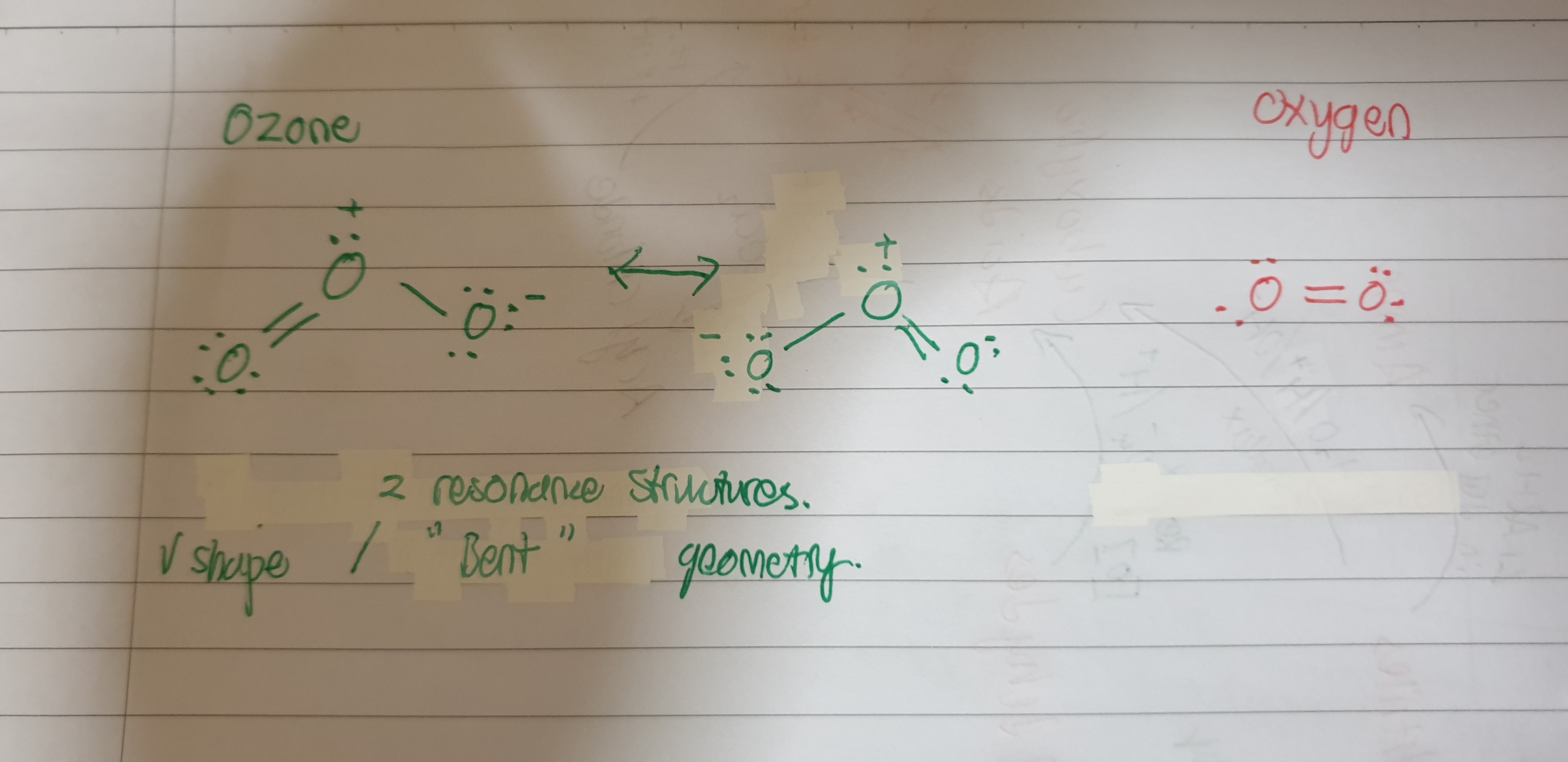
Why does Ozone have a higher boiling point than oxygen? Why is it not for because $O_3$ is polar and has dipole-dipole forces while $O_2$ is non polar and only has London dispersion forces.
Based on the question, I feel that the misconception here is that O$_3$ is polar because of two reasons, its “bent” molecular geometry and the charges on the oxygen atoms. I feel that it is important to mention a concept which is that of resonance. If you refer to the picture, the structure of ozone is neither the structure on the left nor the structure on the right, but in actuality it is somewhere in between, a resonance hybrid of the two structures. Hence, there is no “charge” on any of the oxygen atoms, and there would not be a polar bond because of it.
Instead, what should be looked at is the inherent electronegativity of the elements themselves, and since there is only oxygen in the molecule, there would be no polar bonds. Therefore, the molecular geometry would play no role in the overall polarity of the molecule, and the molecule would still be non-polar. Hence the only intermolecular force which would be present is that of London dispersion forces, and since $O_3$ has a higher Molecular Mass (Mr) as compared to $O_2$, $O_3$ would have a larger electron cloud and hence stronger London dispersion forces, causing it to have a higher boiling point.